Abstract
Primary immune thrombocytopenia (ITP) is an acquired autoimmune inflammatory bleeding disorder with elevated expression of tumor necrosis factor-α (TNF-α) (Pehlivan M et. al. Platelets 2011). In our current study, we isolated the CD14+ monocytes from ITP patients and healthy controls, and performed qPCR assay to test the expression of TNF-α. In ITP patients positive for anti-GPIIbIIIa antibody, monocytes expressed significantly higher levels of TNF-α than that in anti-GPIIbIIIa antibody negative patients or healthy controls, indicating that TNF-α may play an important role in anti-GPIIbIIIa antibody mediated Fc-dependent thrombocytopenia.
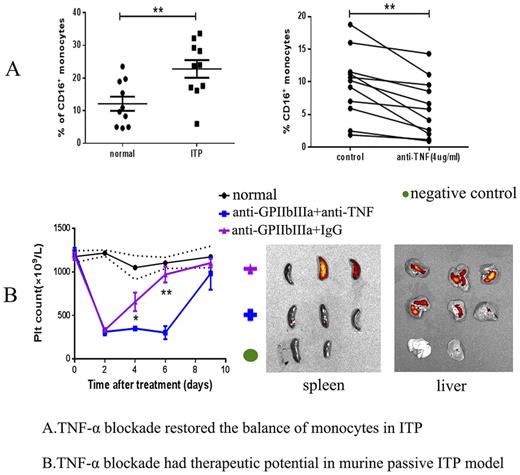
Monocytes and macrophages are the major cell types involved in Fc-dependent platelet clearance in ITP. Monocytes can be divided into three groups based on their CD16 expressions, including the classical (CD14++CD16−), intermediate (CD14++CD16+) and non-classical (CD14+CD16++) monocytes. Macrophages consist of the classically activated M1 type (TNFα+, iNOS+) and the alternatively activated M2 type (CD206+). The CD16+ monocytes (intermediate and non-classical monocytes) and M1 macrophages are pro-inflammatory with increased TNF-α expression. In our current and previous studies, we discovered a deviation of the monocyte/macrophage system manifesting in an increased percentage of intermediate monocytes in the peripheral blood and elevated M1 macrophages in the spleens of ITP patients (Feng Q et. al. J Thromb Haemost 2017). We carried out in vitro blockade of TNF-α using the monoclonal TNF-α antibody in the peripheral blood mono-nuclear cells (PBMCs) of ITP patients and bone marrow-derived M1 macrophages induced by LPS and IFN-γ. We found that blockade of TNF-α could significantly decrease the percentage of CD16+ monocytes and lower the expression of M1 markers like iNOS, TNF, MCP1. We also isolated the classical monocytes from PBMCs and cultured with M-CSF to generate macrophages. Interestingly, CD16 could be expressed by the classical monocyte-derived macrophagesduring the cultivation, while intervention by TNF-α blockade could reduce the CD16 expression during the macrophage-induction process. This finding further indicates the pro-inflammatory role of TNF-a in the monocyte/macrophage system. Subsequently, we performed western blotting to investigate the mechanisms of TNF-α blockade in regulation of the balance of monocytes/macrophages using cultured bone marrow derived macrophages and found TNF-α blockade could induce autophagy in an Akt/PI3K/mTOR-dependent pathway.
To determine the effect of TNF-α blockade on the function of monocytes/macrophages, we performed the phagocytosis assay using FITC-dextran. It was shown that TNF-α blockade could significantly dampen the phagocytic ability of monocyte-derived macrophages from ITP patients and M1 of RAW264.7 cell line. We also isolated the CD4+ T cells from ITP patients and labeled them with CFSE to test the function of monocyte-derived macrophages for stimulating T cell proliferation. The results showed that TNF-α blockade could significantly inhibit the stimulation of T cell proliferation by M1 macrophages.
Finally, in the in vivo study, we established the murine passive ITP model by injecting anti-GPIIbIIIa antibody. We then performed flow cytometry to determine the subtypes of monocytes/macrophages after 7 days of injection. The ITP mice were treated with TNF-α antibody at 5mg/kg or isotype IgG control every other day. We observed that TNF-α blockade could ameliorate thrombocytopenia in the murine model by decreasing non-classical monocytes(ly6c-CD11b+CD115+) from peripheral blood and M1 macrophages(F4/80+TNFα+) from abdomen, liver and spleen. Additionally, we injected the DIR-labeled platelets into those ITP mice and found TNF-α blockade could reduce the rentention of platelets in spleen and liver. Our study showed that TNF-α blockade could decrease the antibody-mediated platelet destruction by restoring the monocyte/macrophage subsets in vitro and in vivo. Therefore, TNF-α blockade might be a promising therapeutic strategy for the management of ITP patients especially those positive for anti-GPIIbIIIa auto-antibody.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal